Why You Should Be Qualified to Conduct Clinical Trials as per ICH GCP
As GCP auditors we keep on facing the same GCP non-compliances when conducting investigator site audits and hosting regulatory inspections due to the sites staff, and also clinical operations personnel overseeing sites, lack of understanding of the seriousness of the procedures they must follow and the impact of their actions on the quality and integrity of the study data.
We wonder so many times, “why was this site selected when it is so obvious, they do not have the adequate resources?”, especially when we have to deal with critical observations and/or serious breaches.
ICH GCP states crystal clear that all staff involved in clinical trials need to be qualified by education, training and experience:
-
ICH GCP Section 4.1 “Investigator´s Qualifications and Agreements” reinforces that before undertaking a clinical trial, it is key to ensure that appropriate qualified staff are available to conduct the trial.
The Principal Investigators (PI) have the responsibility to first verify and confirm that their team members are qualified and trained to discharge their roles in the study, and that their qualifications are documented.
-
ICH GCP Section 5.6.1 “Investigator Selection” specifies that the sponsor is responsible for selecting the investigator(s)/institution(s). Each investigator should be qualified by training and experience and should have adequate resources to properly conduct the trial for which the investigator is selected.
This article clarifies what “to be qualified” means and what are the training expectations for investigators and research site personnel.
What does “to be qualified” mean?
Being qualified requires a combination of education, training and experience.
The education needed for staff working in clinical trials may vary significantly. For example, a laboratory technician may not require a degree if they are only processing samples but may require specific scientific education when performing complex study analyses.
The experience of staff working in clinical trials will also vary noticeably, from a new starter to a very experienced staff member.
The training received should be tailored to the roles and responsibilities being undertaken by an individual
It is also important to keep their training up to date since the regulations, SOPs, tools used, science, medicine, and multiple other factors can change over the course of a trial.
The training should cover:
1. GCP Training
What level of GCP training is necessary?
GCP training supports clinical development of new medicinal drugs by ensuring that site staff:
-
Are educated in the basics of ethical research
-
Understand their roles and responsibilities
-
Conduct their trial related tasks in accordance with regulatory requirements
-
Safeguard the quality, accuracy, and credibility of the data
No person shall conduct a trial otherwise than in accordance with the principles of GCP. Therefore, each person in a clinical trial must receive some training in GCP commensurate with their roles and responsibilities within the trial.
What other type of regulatory training is expected?
The training must not be restricted to training in the local country clinical trial regulations but should also include, as appropriate, training in other available guidance relating to clinical trials (for example, EudraLex Volume 10 or GMP Annex 13 Good Manufacturing Practices for Investigational Medicinal Products training).
Frequency of GCP/ Regulatory Training?
The frequency of GCP and regulatory training is not specified in the regulations; however, it is recommended that training is given at intervals appropriate to ensure staff maintain awareness of GCP requirements, the current country regulations and other applicable regional guidelines.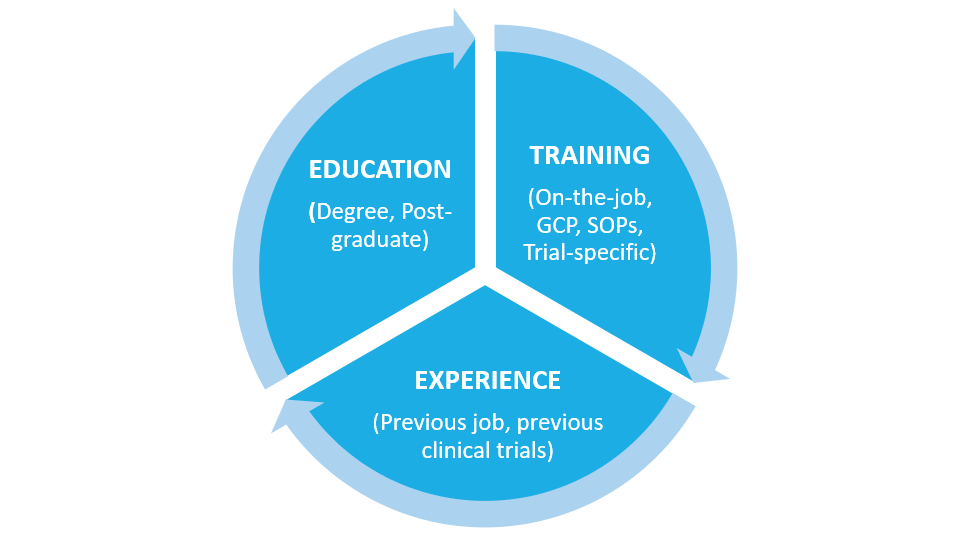
How often this training is repeated is a business decision for the organization/institution concerned. Requirements for the frequency of GCP training should be documented.
Do not forget that training should also be provided in the event that there are significant regulatory updates between scheduled training events.
It is also important to provide a refresher training where, for example, staff have been on extended leave.
View our training on ICH GCP E6 (R2) for Investigators and Clinical Research Staff
2. Training in Written Procedures
Written procedures are the organization’s/institution’s formal Quality System, comprised by policies, Standard Operating Procedures (SOPs), Working Instructions (WI) and associated forms/templates.
The purpose of the written procedures is to ensure that tasks are performed correctly and consistently, and in compliance with regulations and associated guidelines.
The training in written procedures should also be specific to the roles and responsibilities undertaken by a particular staff member.
3. Trial-Specific Training
Trial specific training must be undertaken BEFORE commencing any trial-specific activities.
The training needs to include training in the study protocol and any supporting trial manuals and guidance necessary for each role. For example: training in the trial indication, study specific assessments to be performed and their timeframe, clinical systems used such as eCRF, IXRS, ePRO devices.
All staff must receive an appropriate level of training to allow them to perform their trial-related duties, taking into consideration training needs for staff who join a trial team after the trial has started.
Qualifications evidence
The investigators and other site staff that are producing data on the study must provide documented evidence of their qualifications by a current CV, training records and other documentation requested by sponsors, ethics committees, or regulatory authorities.
What records should be maintained?
The extent and content are the institution´s decision, but typically the following records should be maintained to demonstrate staff qualifications:
-
Current job description
-
An up-to-date CV demonstrating current and previous education and experience
-
Confirmation of GCP training taking place through a training certificate which refer to the framework used in the training. For example: ICH GCP E6 (R2), EU Directives
-
Documented training in written procedures, relevant to the individual’s duties and clinical trials responsibilities
-
Documented trial-specific training, relevant to the individual’s role and responsibilities in a specific trial
-
Documented competency sign-off for specific assigned tasks requiring it
All these documents define each team member qualifications and support their role in the trial.
Conclusion
Training staff working on clinical trials, takes a whole other dimension due to the human risk implications. It is a must that all staff involved in the conduct of clinical trials are qualified to safeguard the rights, safety and well-being of trial subjects and to guarantee the quality and integrity of the study data.
When sponsors decide to select research-naive sites, they need to deal with the risks that it implies and implement mitigating actions before it is too late. Frequent training and close supervision are critical for those inexperienced sites.
If a PI decides to delegate trial tasks to inexperience site personnel, they need to be supplemented with more experienced site staff and ensure they receive adequate training tailored to their responsibilities.
Also consider that all site staff, whether research-naive or experienced, need to receive frequent training on GCP requirements. The training needs to be effective, efficient and engaging so it contributes to the learning process and to change behaviors.
Most of the time those trainings lack details regarding daily site activities and/ or the potential impact of GCP non-compliance on the study subjects and data. Remember that the most relevant moment is not the moment of training, but the moment of execution and if the training content is not practical enough and it does not include the right scenarios, it will be forgotten and consequently useless.
We have created an engaging ICH GCP E6 (R2) online course covering the training topic amongst other critical areas that investigators and other clinical research staff conducting clinical trials need to properly understand…
Click here to view this course
References
-
Integrated Addendum to ICH E6 (R1): Guideline for Good Clinical Practice E6(R2). November 2016.
-
MHRA Good Clinical Practice Guide, Medicinal and Healthcare products Regulatory Agency, UK



