Validation and Qualification of eClinical Systems (2) – Contracts/Agreements
After the selection of a vendor, appropriate contracts should be put in place prior to the commencement of work. Contracts should clearly detail the delegated tasks, duties and functions between the parties, and the required standards of service.
INTRODUCTION
The EMA “Notice to sponsors on validation and qualification of computerised systems used in clinical trials” published on 7th April 2020 covers two main aspects: documentation of qualification activities which we explained in a previous publication, and the contractual arrangements with eClinical systems vendors which is the scope of this article.
There are many types of contracts/agreements such as for example. Service level agreements (SLA), master service agreements (MSA) with associated work orders (WO). Sponsors and vendors may each have their own templates and agreement requirements. In order to ensure the requirements of each party are met, a process for the development and execution of contracts should be formalized
Clear, written agreements should be in place to document any arrangements between the sponsor and the vendor with regards to qualification and validation before the delegated tasks are initiated. The sponsor remains responsible for ensuring that the conduct of the trial and the final data and data that are submitted to support a Marketing Authorization Application (MAA) comply with relevant legislation.
eCLINICAL SYSTEMS VENDOR CONTRACTS
During GCP inspections an increasing amount of deviations from GCP standards have been identified by the inspectors in view of sub-standard contractual arrangements and related procedures. These deviations could be prevented by improved contracts between sponsor and vendors of eClinical systems.
The key processes and/or components that sponsors should contractually ensure are explained below:
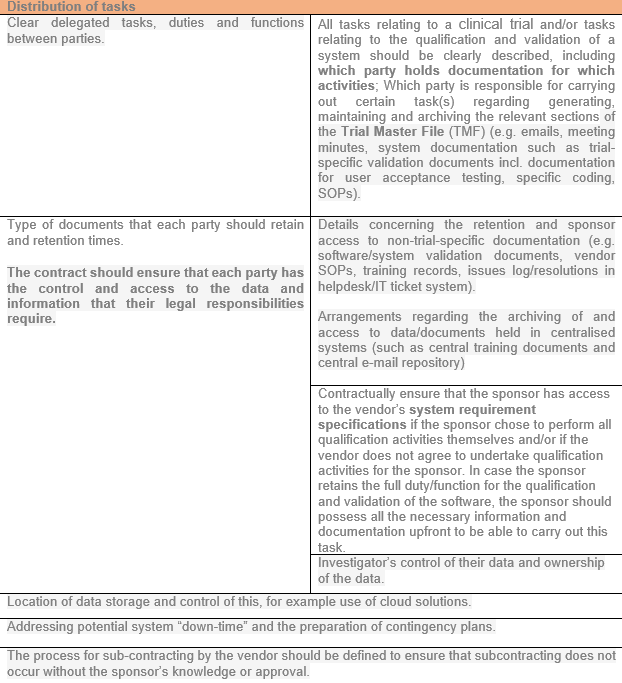
Distribution of tasks
Standards to be followed

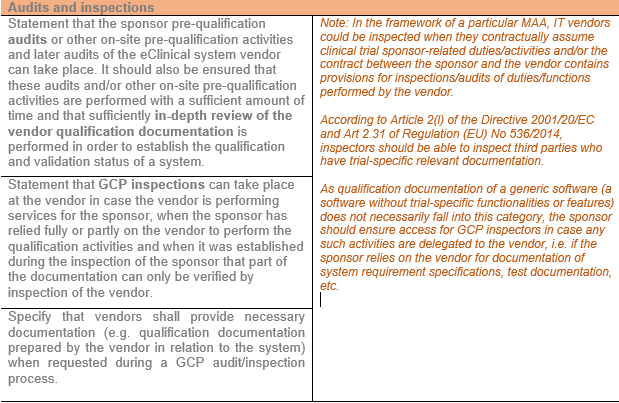
Audits and inspections
Serious Breaches

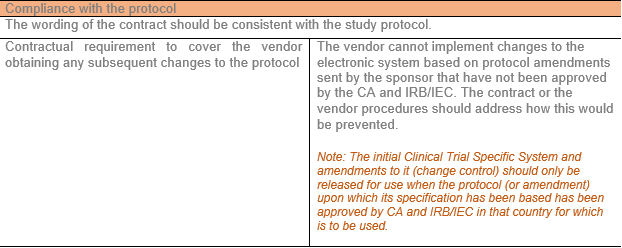
Compliance with Protocol
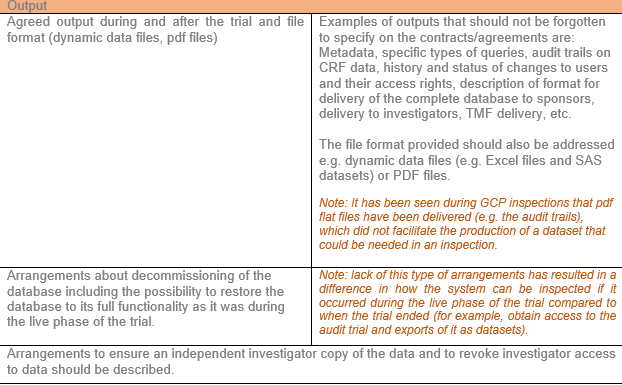
Output
OVERSIGHT OF CONTRACTS
Once a contract is executed, processes should ensure that contracts remain current and that the requirements of the contract are being met by all parties. It is also important that everyone with contractual obligations is aware of and has access to the contract.
Oversight of contracts can be achieved by planning periodic reviews of the contracts or by defining specific triggers for the review, such as protocol amendments, updates to relevant legislation or changes to the quality system.
CONCLUSION
If appropriate contracts cannot be put in place, for example because a vendor does not allow provision of adequate measures as described throughout the article (access to system requirements specifications, prequalification audits, access for GCP inspectors, etc.), systems from such a vendor shall not be used in clinical trials.
Sponsors may transfer any or all of the sponsor’s trial-related duties and fucntions to vendors, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor.
Remember that you can now access our ICH GCP E6 (R2) for Investigators and Clinical Research staff online course below:
👉 JOIN NOW: ICH Good Clinical Practice (GCP) E6 (R2)
REFERENCES
-
International Council for Harmonization (ICH): ICH Harmonized Guideline, Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). Step 4, version 9 November 2016.
-
EMA. Notice to Sponsors on validation and qualification of computerised systms used in clinical trials. April 2020.
-
EMA Website. GCP Q&A section (Q&A #9)
-
MHRA. GCP Symposium. Computer System Validation, A.Fisher. February 2019, London (UK)
-
MHRA. Good Clinical Practice Guide. 2012
Author: Dr Leire Zúñiga, Director and Principal GCP Consultant
PHARMITY, 19th of May 2020
www.pharmity.com



