Advanced Therapy Investigational Medicinal Product (ATIMP) Trials
On Friday 12th April 2019, I had the opportunity to attend a seminar on good cellular therapy practices organized by the Spanish Ministry of Health and the Spanish Competent Authority, Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) representing our consulting firm, Pharmity.
Speakers from the Ministry of Health, AEMPS, the Cell Theraphy Network (TerCel) and patients’ associations gave an overview of the current status of ATIMP in Spain and Europe with special focus on cell therapy.
It was highlighted on multiple occasions during the seminar, that Spain is the leading country in clinical research with advanced therapy medicinal products (ATMP) (800 clinical trials were authorized in Spain in 2018) and that clinical trials are fundamental tools to make these medicines available to patients.
This article will summarize the overarching principles in ATIMP/ATMP regulations and guidelines relevant to Good Clinical Practice (GCP) that were not covered in detail during the aforementioned seminar.
What is an ATIMP?
The Spanish Royal Decree (RD) 1090/2015 defines “ATIMP” as an investigational medicinal product which is an advanced therapy medicinal product as defined in point (a) of article 2(1) of Regulation (EC) No 1394/2007:
‘Advanced therapy medicinal product (ATMP)’ means any of the following medicinal products for human use:
- a gene therapy medicinal product as defined in Part IV of Annex I to Directive 2001/83/EC,
- a somatic cell therapy medicinal product as defined in Part IV of Annex I to Directive 2001/83/EC,
- a tissue engineered product as defined in point (b)1.
Important consideration should be given to the classification and definition of the medicinal investigational product at its very early stages of development. Companies can consult AEMPS to determine whether the medicine they are developing is an advanced therapy medicinal product (ATMP). The criteria for ATMPs are set out in Regulation (EC) nº 1394/2007 (Article 17)2.
Legal Framework for ATMPs in EU and implementation in Spain
The main regulation for ATMPs in the European Union (EU) consists of Regulation (EC) No 1394/2007 which was developed as a lex specialis of Directive 2001/83/EC3 to provide the regulatory framework specific for ATMPs in terms of authorization, supervision and pharmacovigilance.
The specifics of ATMPs were implemented into other legal texts and guidelines by the European Commission (EC). The scientific and technical requirements specific to ATMPs concerning quality, safety and efficacy were introduced by the Commission Directive 2009/120/EC4 to amend Annex I of Directive 2001/83/EC.
The Annex 2 of the EU guidelines for Good Manufacturing Practice (GMP)5 was updated by the European Commission with respect to the specific requirements of ATMPs to comply with Article 5 of the ATMP regulation and a guideline on good clinical practice to ATMPs was developed.
Furthermore, other scientific and procedural guidelines and guidance documents for ATMPs have been developed by the European Medicines Agency (EMA).
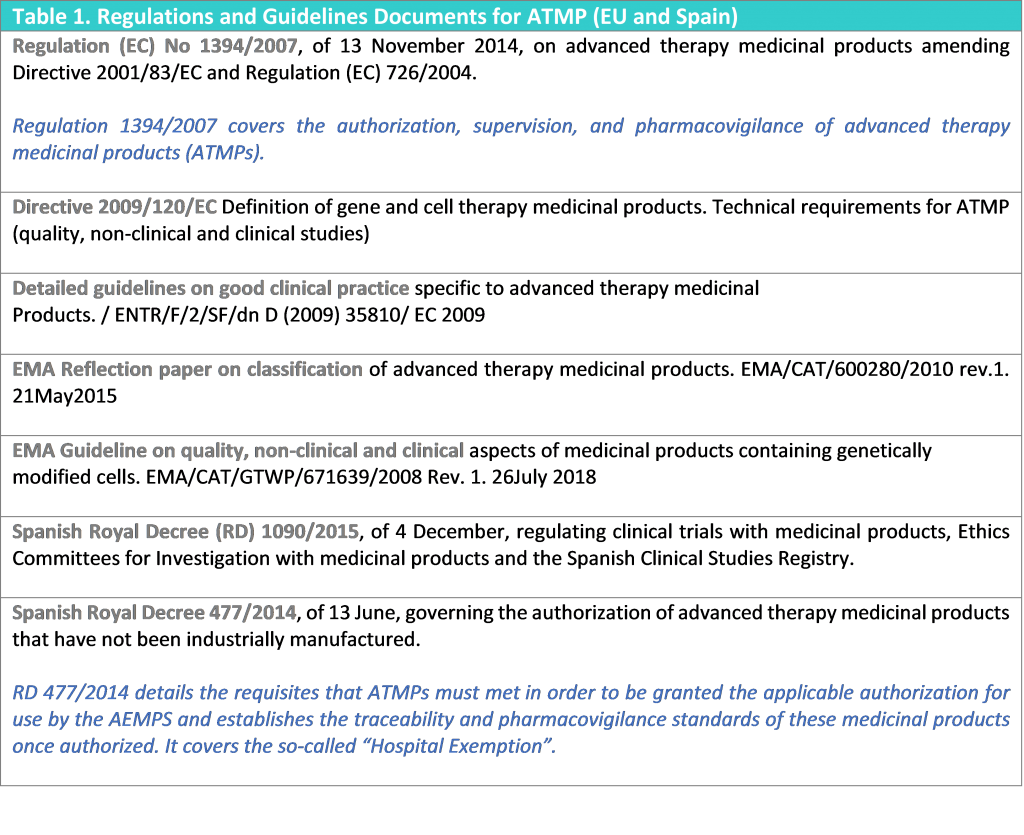
Table 1 lists all the aforementioned EU documents and the main legislation and reference guidance for ATMPs in Spain.
There are three main types of authorization that can be granted in Spain:
- Clinical Trials (AEMPS). The national competent authority (AEMPS) when assessing the request for authorization of a clinical trial involving an ATIMP consider in particular the adequate traceability arrangements and the follow up strategy, including the definition of the end of the trial and risk-assessment5.
- Compassionate Use (AEMPS). It is not possible to have patients under compassionate use if there are no clinical trials supporting the evaluation.
- Authorization for use (not for commercialization) (AEMPS) (Royal Decree 477/20146)
- Hospital Exemption. A regulatory option covered by RD 477/2014 for unauthorized advanced therapy medicinal products.
Regulation (EC) nº 1394/2007 specifies that “Advanced therapy medicinal products which are prepared on a non-routine basis according to specific quality standards, and used within the same Member State in a hospital under the exclusive professional responsibility of a medical practitioner, in order to comply with an individual medical prescription for a custom-made product for an individual patient, should be excluded from the scope of this Regulation whilst at the same time ensuring that relevant Community rules related to quality and safety are not undermined”1. Each EU member state regulates the medicine products that have not been industrially manufactured. In Spain this is regulated by Royal Decree (RD) 477/20146.
- Marketing Authorization (EMA). Centralized marketing authorization procedure coordinated by the European Medicines Agency (EMA). One marketing authorization is granted valid for all EU member states
Special Considerations for ATIMP trials
Clinical Trials using ATIMPs are conducted under the same clinical trials regulations as all other clinical trials; however, such trials shall additionally comply with the guidelines for good clinical practice which are specific for advanced therapy medicinal products (Table 1. Regulations and Guidelines for ATMP).
The overarching principles related to ATIMP clinical trials are as follows5:
- The use of each ATIMP should be traceable through the sourcing, manufacturing, packaging, storing, transport, delivery to the hospital/institution/private practice, administration to the subjects, reconciliation and destruction or final disposition.
- The number of links in the chain of custody (from donation to subject application) should be no more than necessary. If the product or part of it originates from a donor, records should contain sufficient detail to allow linking from the donor to the individual subject who received the product and vice versa; however, personal data should be protected by use of anonymous coding system so that neither donors nor recipients remain identifiable.
- The protocol should specify the follow-up (FU) period for subjects who should be followed-up during and, if necessary, after the end of the clinical trial both for their own care and to allow data collection as needed. The nature of FU and, if applicable, long-term FU after the end of the trial should be determined based on the nature of the ATIMP, the current state of knowledge regarding that ATIMP and a risk analysis. Processes should be established to enable contact with subjects to be maintained throughout the required FU period.
- The medical care given to, and medical decisions made on behalf of, subjects should always be the responsibility of a qualified physician or a qualified dentist. However, there may be circumstances where a representative of the sponsor – experienced in the administration of the ATIMP – needs to be present during the application of the ATIMP to the subject. This expert may provide advice and information to the investigator/responsible physician, but the investigator/responsible physician remains responsible for any decision to halt or modify the application procedure.
Risk analysis
The sponsor should ensure that an ongoing risk analysis, based on existing knowledge of the type of product and its intended use, is performed and provided to the investigator involved in a clinical trial with that ATIMP, through the investigator brochure or updates to it and to the patient through the informed consent or updates to it.
The risk analysis for each ATIMP clinical trial should contemplate, among other things, the need for, the duration and the nature of follow up5.
Traceability of ATIMP
Where an ATIMP contains human cells or tissues, the sponsor of the trial, the manufacturer and the investigator/institution where the product is used should ensure that there is a traceability system in place to link the donor/source to the donation, to link the donation to the product and then to link the product to the subject. The traceability has to work in both directions from source to subject and from subject to source.
GCP contains requirements for accountability of IMPs that contributes to the traceability of the ATIMP from the manufacturer onwards.
Traceability requirements should also be compatible with the requirements in Annexes 2 to the GMP Guidelines7 (Manufacture of biological active substances and medicinal products for human use) and to the GMP Guidelines as amended for the specific requirements of ATIMPs.
The guideline on traceability referred to in Article 15(7) of the Regulation No 1394/2007 should also be applicable to clinical trials on ATIMPs.
In the event that the clinical trial is suspended or prematurely ended or the product development discontinued, the sponsors retain their obligation to ensure that the traceability system is maintained (including where the ownership of the ATIMP is transferred to another legal entity; the new owner should take responsibilities for maintaining the traceability). In case of bankruptcy or liquidation of the sponsor, and in the event that the ownership is not transferred to another legal entity, the relevant traceability records related only to ATIMPs shall be transferred to the national competent authority.
The traceability procedures and the documentation process should be described in the clinical trial protocol and amended as needed which is sent to the competent authority for approval.
The system should allow full traceability from the donor to the recipient through anonymous coding systems.
Informed Consent
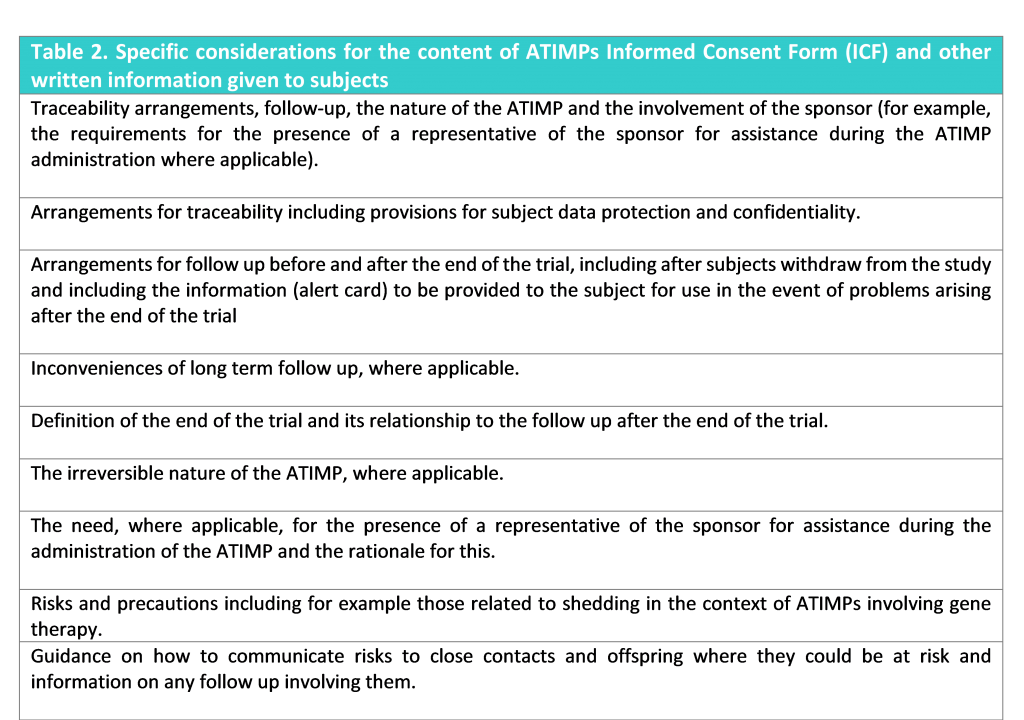
There are specific considerations for consent for ATIMPs. The subjects and their legal representatives as applicable, should be informed by the investigator of the specific issues arising from ATIMPs (Table 2).
Essential Documents
As regards to the keeping of records, the rules as set out in Directive 2001/20/EC8 and 2005/28/EC9 apply. The traceability records should be kept by the sponsor of the trial and investigational sites for a minimum of 30 years after the expiry date of the product, or longer if required by the terms of the clinical trial authorization or by the agreement with the sponsor (this requirement differs for ATIMP as compared with other medicinal products).
Before, during and after completion or termination of the trial, each party should hold the necessary information available at all times to ensure bidirectional traceability, linking the donor information at the procurement site to the ATIMP and the clinical trial subject information at the clinical trial site to the ATIMP, whilst ensuring the data protection legally required for both the donor and the clinical trial subject.
Safety Reporting
The requirements for notification of adverse events and adverse reactions by the investigator and the sponsor in the context of clinical trials are laid down in Articles 16 and 17 of Directive 2001/20/EC and their implementing guidelines. New events related to the conduct of the trial or the development of the ATIMP and likely to affect the safety of the subjects such as serious adverse events (SAEs) associated with trial procedures, and significant hazard to the subject population should be reported according to the existing timelines for expedited reporting (this requirement differs for ATIMP as compared with other medicinal products).
The sponsor should provide information and training to the investigator on any additional protocol and/or product specific requirements for the reporting of adverse events and this reporting process should be outlined clearly in the clinical trial protocol. Table 3 details the safety issues that are of special concern for ATIMPs.

Differentiated causality assessment concerning the safety issues mentioned above should be described in the clinical trial protocol and implemented in the adverse event reporting system.
Long-term Follow-Up
The need for, the duration and the nature of follow-up should be determined by the sponsor for each clinical trial based on the nature of the ATIMP, the current state of knowledge regarding that ATIMP and a risk analysis, including the risk for close contacts and offsprings.
The sponsor may wish to discuss the duration of follow up with the competent authority, AEMPS. This follow up should be aligned with the specific requirements of the product under development, should be described in the protocol, and amended as needed in accordance with the evolving experience with the ATIMP and should make it clear which follow up activities should take place prior to and after the end of the clinical trial. The rationale for the chosen follow-up, including the available supporting information, should be documented and kept as an essential clinical trial document.
The follow up should be considered from the following aspects:
- For the protection of the subject (clinical follow up).
- For the purpose of collection of specific data which might not involve all subjects (safety follow up and efficacy follow up).
All subjects participating in a clinical trial with an ATIMP should receive from the investigator an alert card, which has been previously agreed by the sponsor and approved by the Ethics Committee, containing as a minimum; the name of the subject, the investigator contact number and information regarding the medical treatment received.
Subject alert cards should inform treating physicians about the product used and any other source of data available in case of safety/efficacy issues, and of the need to inform the competent authority, the investigational sites and the sponsor in the event of certain serious adverse reactions.
The protocol should define the end of the trial and which follow up should take place after the end of the trial. The safety and efficacy follow up involving active data collection (study visits) should form part of the clinical trial whereas clinical follow up and passive data collection may take place after the end of the trial.
Where follow up after the end of the trial is required, in particular when this occurs over a long term, the sponsor needs to ensure that there is a process in place for follow up of the subjects treated with the product – even in cases where the product development is discontinued or the (former) sponsor ceases to exist as a legal entity. This process should be described in the protocol, which should be amended as needed.
Where a subject is withdrawn from a trial at their own request or based on a decision of the investigator, the follow up should be maintained depending on the consent of the subject.
Where to find information regarding ATIMPs in Spain?
Table 4 lists the main sources where information regarding ATMP/ATIMPs can be located in Spain.

Conclusion
The EU legal framework for ATMPs sets high regulatory standards, including manufacturing in compliance with GMP and the centralized procedure for obtaining a marketing authorization, which requires among others, conducting clinical trials. This puts an enormous regulatory burden on the manufacturers of ATMPs, who are mainly represented by academic institutions with limited regulatory expertise, budget and staff.
The development, regulation, and clinical use of the vast majority of ATMPs are still evolving. As a consequence, defining appropriate regulatory standards taking into account the complexities inherited in these products is crucial.
For the field to advance, developers and regulators need to collaborate to continuously monitor and evolve methodologies and regulations for ATMPs. This was a clear message sent by AEMPS during the seminar on cell therapy that took place last 12th April 2019 in Madrid.
If medicines traceability is critical, for ATMPs it takes higher priority. Traceability along with an ongoing risk-assessment (and risk minimization strategies) and a well-established post-marketing surveillance are fundamental pillars when talking of ATMPs.
References
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004.
- Reflection paper on classification of advanced therapy medicinal products. EMA/CAT/600280/2010 rev.1. 21May2015
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use
- Commission Directive 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use as regards advanced therapy medicinal products
- Detailed guideline on good clinical practice to advanced therapy medicinal products. European Commission. December 2009
- Spanish Royal Decree 477/2014, of 13 June, governing the authorization of advanced therapy medicinal products that have not been industrially manufactured.
- Eudralex Vol 4 EU guidelines for GMP, Annex 2 Manufacture of Biological active substances. European Commission.
- Commission Directive 2001/20/EC of 4 April 2001, relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use.
- Commission Directive 2005/28/EC of 8 April 2005, laying down principles and detailed guidelines for good clinical practice as regards investigational medicinal products for human use, as well as the requirements for authorization of the manufacturing or importation of such products.
Author: Dr Leire Zúñiga, Director and Principal GCP Consultant
PHARMITY, 22nd April 2019


